CMS Lymphedema Compression Coverage Resource Guide
Everything you need to know about the Lymphedema Treatment Act, all in one place.
Updates You May Have Missed on the CMS Compression Guidelines
CMS has released their final rule regarding the implementation of the Compression Guidelines which outlines how they intend to begin covering compression garments and related accessories for patients diagnosed with lymphedema. The full 65-page document can be viewed here (beginning on page 350). Below is a summary of some of the key provisions of the final rule:
Covered items: The following items will be covered by Medicare beginning Jan. 1, 2024, for patients diagnosed with lymphedema:
- both standard and custom-fitted gradient compression garments
- gradient compression wraps with adjustable straps
- compression bandaging systems
- other items determined to be lymphedema compression treatment items
Frequency limitations: Medicare will cover:
- Three daytime garments or wraps with adjustable straps for each affected limb or area of the body, replaced every six months.
- Two nighttime garments for each affected limb or area of the body, replaced once every two years.
- *These limitations are higher than what was originally suggested in the proposed rule, and industry stakeholder comments played a key role in raising the frequency limitations that were finalized.
HCPCS Codes: There are a couple tables contained within the final rule, namely tables FF-A 1 and FF-A 2, which outline the codes that will be used to represent many of the compression-related products used to treat patients with lymphedema.
- CMS is keeping several of the existing compression codes that several of you may already be familiar with (A6530-A6549 range). These are outlined in Table FF-A 1 on page 372.
- CMS is creating 57 new HCPCS codes to better represent additional lymphedema-related compression products. These new HCPCS codes have yet to be determined, but the descriptions of each new code can be found in Table FF-A 2 on page 389.
- CMS is creating nine additional new A-codes to replace nine existing S-codes (S8420-S8428) that currently represent compression sleeves, gloves, gauntlets, wraps, and bandages.
Pricing/Reimbursement: CMS has finalized the full fee schedule for 2024 and has included in that fee schedule several new HCPCS codes and corresponding reimbursement rates for compression garments and related accessories. The full 2024 DMEPOS fee schedule can be found here. We have also included a separate resource that only highlights the compression garment and related accessory codes for this new product category.
855S Application Released:
For those businesses that have been patiently waiting for the 855S application to be updated with the Lymphedema Compression Treatment items as a product being offered, it is finally here.
While the electronic version in PECOS was updated a while back, there are suppliers that still use the hardcopy of the 855S and can now update the products and services being offered with the LCT items.
During the waiting process, instructions were to notify the accreditation organization and then update the enrollment contractor once the 855S was released.
If you are only adding the LCT items (S04) as a product being offered, the first step is section 1A which then instructs to go to section 1B:
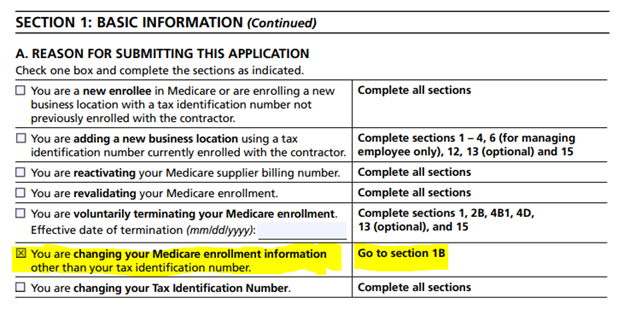
The second step is section 1B, selecting products and services. Then following the sections listed in the adjacent column for completion include Section 2, where the new product category is listed.
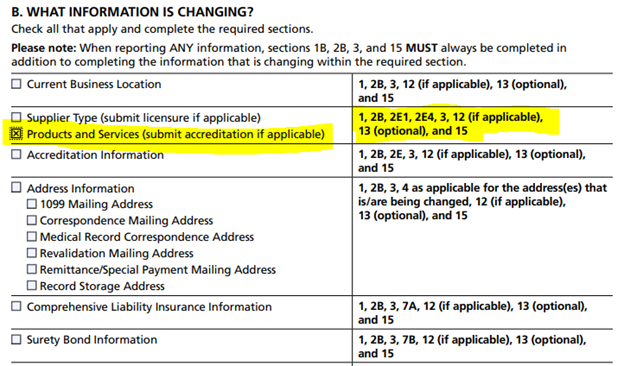
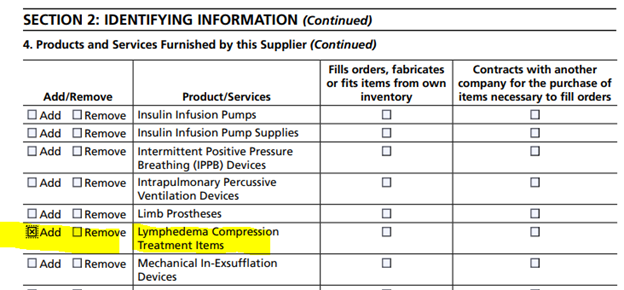
Once all the indicated sections have been completed, suppliers then submit to the appropriate provider enrollment contractor. This is also a good time to communicate with the accreditation organization about the updated application.
NPE West are any states west of the Mississippi River: Palmetto GBA
NPE East are any states east of the Mississippi River: Novitas Solutions
You can submit any additional updates with the application, if necessary. Just be sure to follow the instructions listed.
Any questions can be submitted to rcs@vgm.com.
- Lymphedema Compression Treatment Items – Correct Coding and Billing - CGS
- Lymphedema Compression Treatment Items
- Discussion Board - the VGM members-only portal features a discussion board where members can ask each other advice, questions, or best practices regarding the compression guidelines. Start your own discussion post or discover previous discussions to find out what other members are asking.
